Oncology
Management of
vascular access
Oncokit®
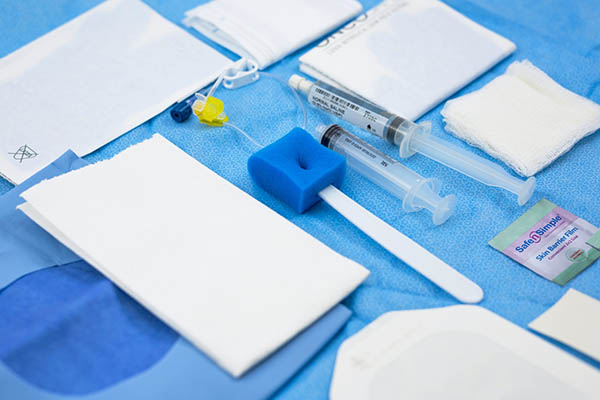
The OncoKit® Venous Access is a kit that integrates oncology solutions for fully implanted catheter access as suggested by the Centers for Disease Control and Prevention (CDC), Oncology Nursing Society (ONS) and Infusion Nursing Society (INS) guidelines. OncoKit® Venous Access converges on the search for oncology service for an effective bundle for maintaining the fully implanted catheter as it brings together the best and safest devices in a single sterile package.
- Standardization of the fully implanted catheter handling procedure
- Maximum barrier bundle against fully implanted catheter infection
MiniLoc®
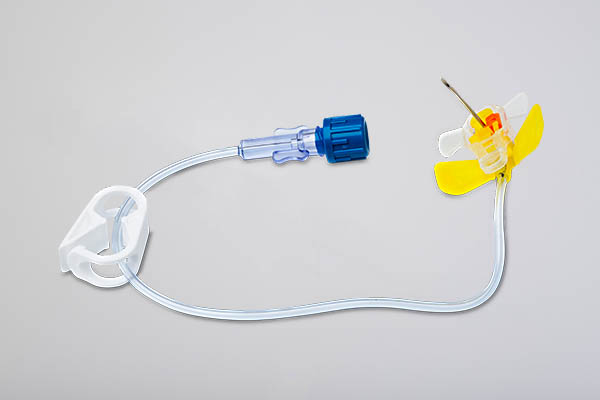
Differentiated by the ergonomic handle and siliconized needle, the MiniLoc®, huber needle, has integrated security system given NR 32, which garante safety forto the professional.
- Butterfly system ergonomic handle for added firmness
- Easy-to-operate integrated safety device with audible confirmation
- Non-coring siliconized needle providing less puncture pain
- Latex and DEHP Free
- Meets NR 32
SafeStep®
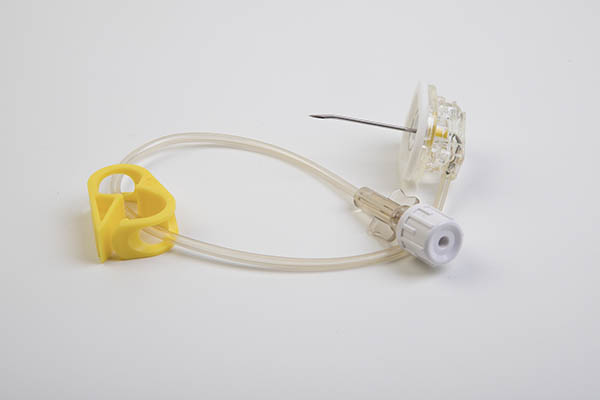

The huber SafeStep® needle is distinguished by its low profile, smaller base and comfort pad for the patient. SafeStep also meets the requirements of NR 32, ensuring safety for the professional.
- Low and compact profile
Transparent base for insertion site visualization - Comfort pad, non-absorbent
- Easy-to-operate integrated safety device with audible confirmation
- Latex and DEHP Free
- Meets NR 32
Power loc®
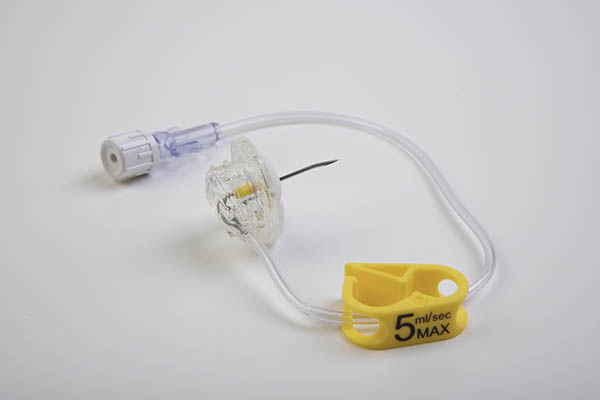
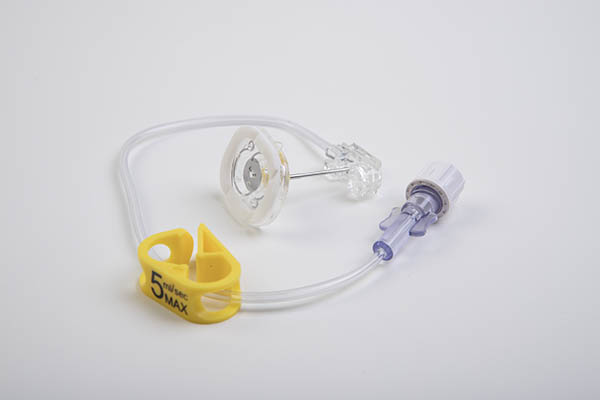
The Power Loc® is the huber needle with integrated safety system for high flow infusion, for example contrast infusion, which requires high pressure infusion of 5 mL / s (300 psi).
- Contrast infusion compatible by injection pump
Identification of flow capacity printed on clamp - Low and compact profile
Transparent base for insertion site visualization - Comfort pad, non-absorbent
- Latex and DEHP Free
- Meets NR 32
PraxiJect ™
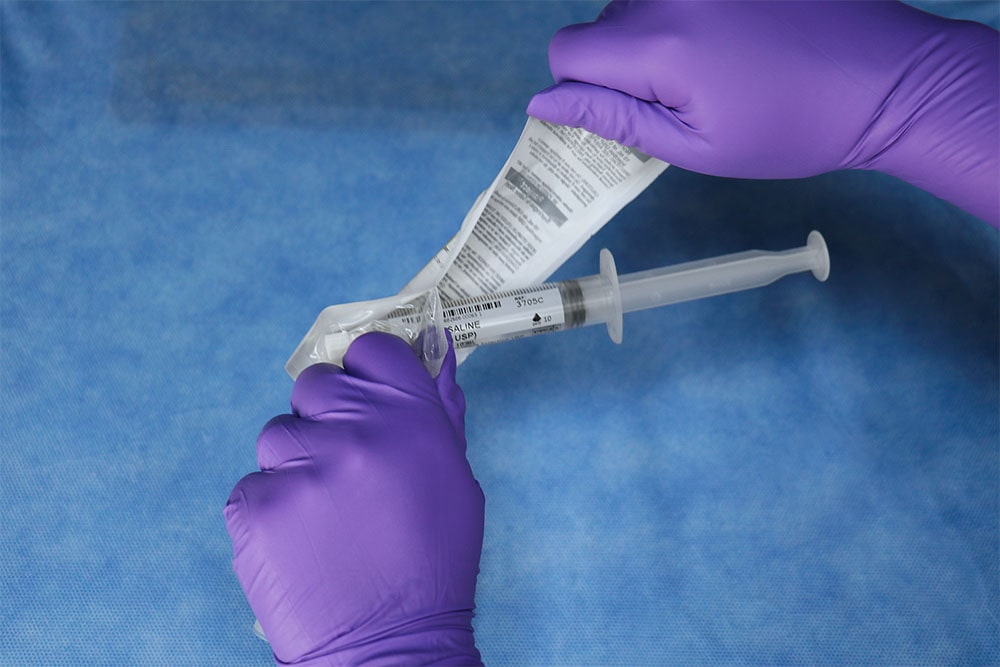
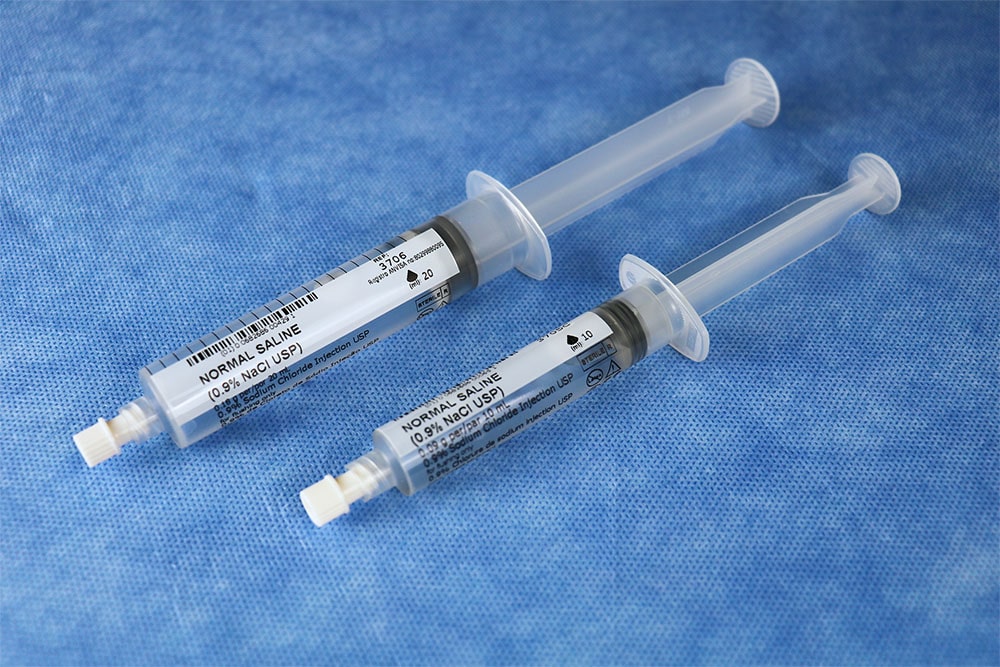
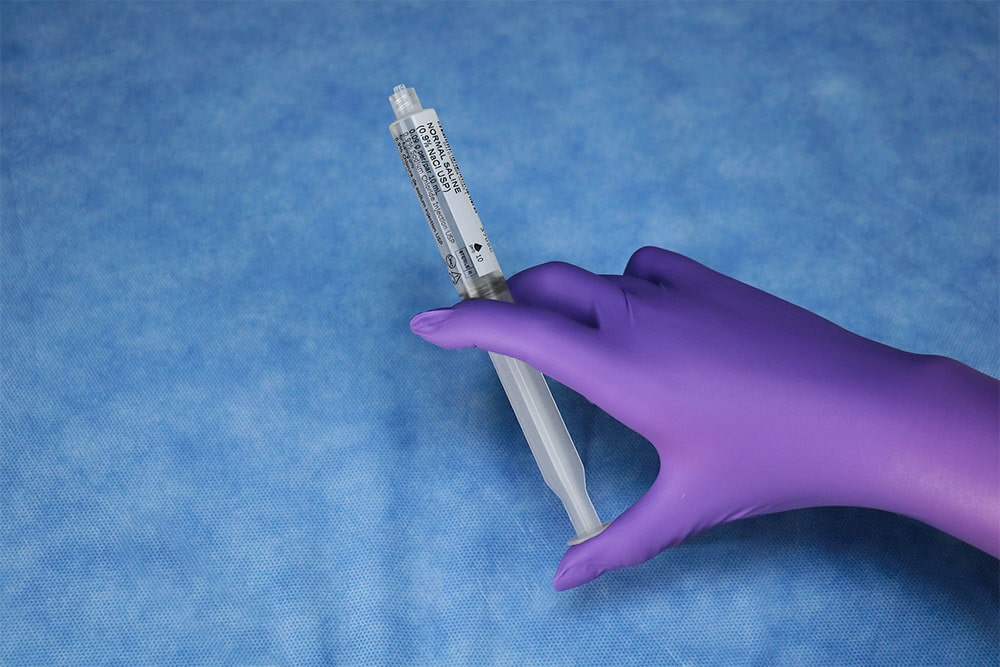
PraxiJect ™ is the only sterile saline-filled syringe inside and out and meets the requirements of the US Pharmacopoeia recommended for sterile field use for flushing and locking venous access devices.
- Increases security
- Catheter Cleaning
- Catheter Flushing
- Decreases catheter damage
- Reduce waste and costs
- Minimizes administration errors
ivKIT®
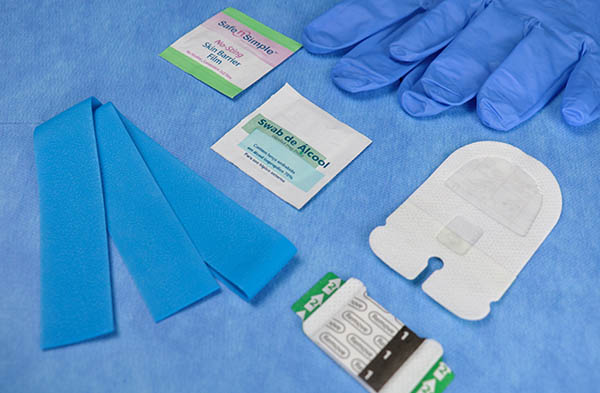
By combining the best and safest devices in a single sterile package, the ivKIT® Peripheral Vascular Access Kit assists in the construction of the safe peripheral puncture bundle. IVKIT meets Anvisa's recommendations for safe peripheral access handling.
- Standardization of the procedure
- Agility to the nursing staff
- Convenience for the service
- Maximum barrier against infection
ivKIT® Guided Puncture

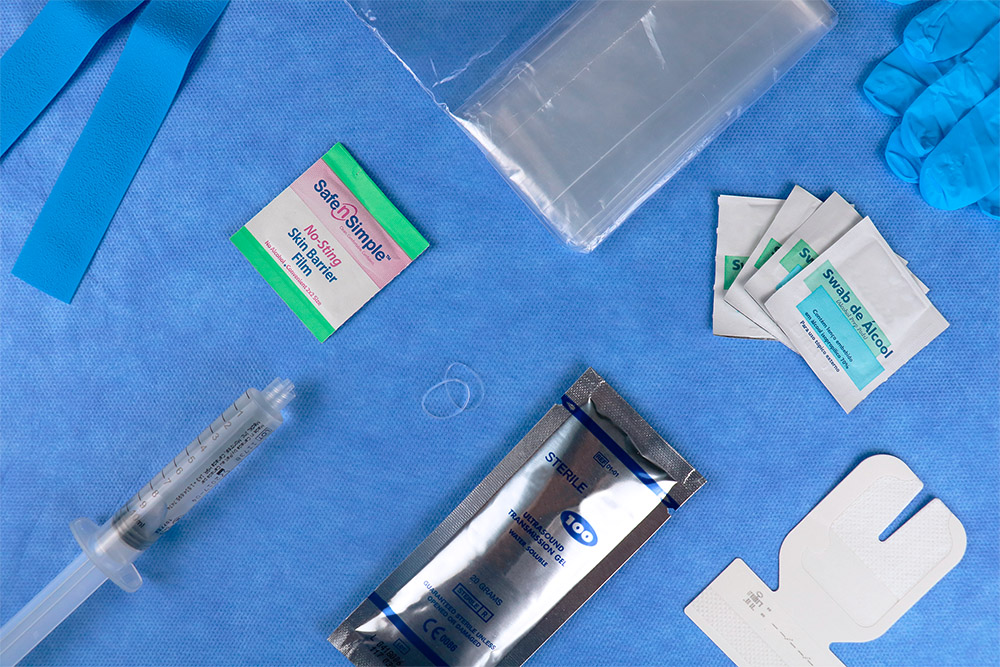
The proposal of ivKIT® Peripheral Access for Guided Puncture is to support the assistance team in guaranteeing the execution of the aseptic technique, offering a single sterile package with the necessary materials, so that the professional can decide and manage the best selection of vein and device, preserving the blood vessel and ensuring the safe route of administration.
- Standardization of the procedure
- Agility to the nursing staff
- Convenience for the service
- Maximum barrier against infection
OncoSafe® Neutral®




The OncoSafe® Neutral is a neutral displacement connector that acts in the creation of the closed system. The function of the device is to reduce the risk of system contamination and infections associated with venous access devices.
- Compact
- Luer Lock connection
- Neutral Displacement (Prevents backflow of blood into the catheter, preventing occlusions from occurring and reducing the risk of infection)
- Completely transparent connector
- Flat and smooth septum
- Sealing ring