Our company
BMR medical
BMR Medical has been a manufacturer of health products since 2005. With the mission of developing high quality and technological products, the manufacturer from Paraná, BMR Medical, has products for oncology, hematology, analgesia, digestive system surgery, plastic surgery, vascular surgery , dermatology, gynecology and urology.
In line with the national policy for the development of the health industrial complex, BMR Medical built its manufacturing unit located at BR 116, nº 1440, at km 1.4, the highway that connects Curitiba (PR) to São Paulo (SP). The industrial plant has six thousand square meters of built area, out of a total of 37 thousand square meters.
To guarantee the safety and effectiveness of the products it offers, BMR Medical has the most important international and national certifications, such as the North American FDA, European Community and Anvisa. The company is also a member of the Brazilian Association of Importers of Medical and Hospital Equipment, Products and Supplies (ABIMED) and the Brazilian Association of the Medical, Dental, Hospital and Laboratory Articles and Equipment Industry (ABIMO).
Industry
- Representation and distribution of health products in Brazil and Mercosur;
- Registration of health products with health surveillance agencies in Mercosur;
- Assistance in regulatory affairs;
- Assistance in clinical studies in Brazil;
- Licensing of new technologies and products;
- Development of new products.
Present in Latin America and Europe, Paragon technology demonstrates the ability of BMR Medical, ISO 13485 certified, to manufacture and supply products with national and international quality standards. Paragon technology was the first developed in the world to provide quality of life and allow the patient to receive treatment in the comfort of their home, dispensing hospitalization. The benefits of this technology are available for treatment with antineoplastic drugs, painkillers, anbitiotics, iron chelation therapy.
The industry excels in individual and corporate attitudes of environmental responsibility to ensure a cleaner and healthier future for future generations. Among employees, we encourage conscious consumption of water, energy, proper disposal and, whenever possible, recycling of waste. In addition, we give priority to the use of transport systems with the lowest pollution possible. To recognize and respect the individualities and potential of each employee, the industry was built respecting accessibility standards, with ramp, elevator, etc.
- Medical compressed air plant, free of particles, oil and water.
- ISO class 7 in production areas and ISO class 8 in other environments.
- Self-leveling epoxy floor, following the drug industry standard.
- Partitions in thermal insulating material with visors that give visibility and comfort to the productive areas.
- Rounded aluminum corners.
- All environments are monitored by cameras and access to production areas is restricted by automated system.
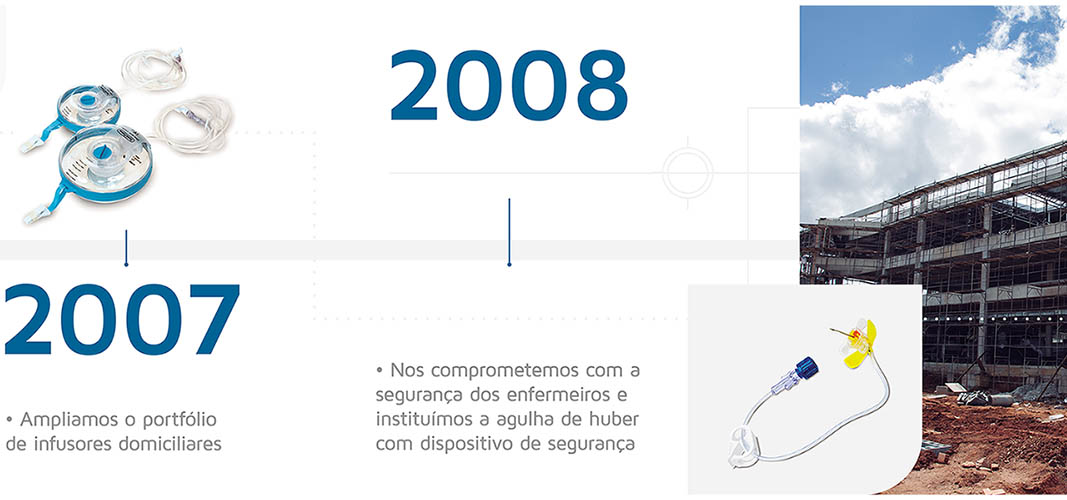
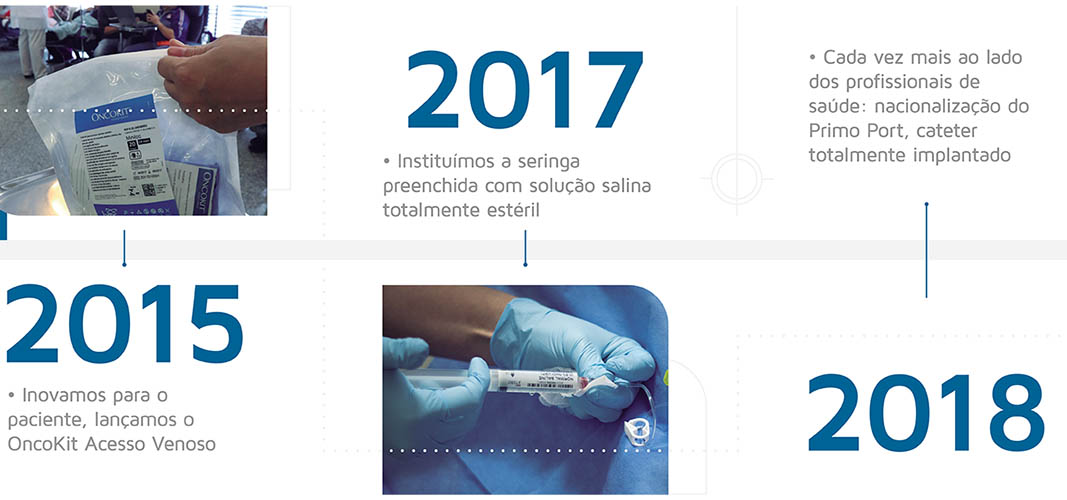
Timeline